Contents
Bicarbonate bincike
Ma'anar bicarbonates
The ions bicarbonates (HC03-) suna cikin jini: suna taka muhimmiyar rawa a cikin pH tsarin. Su ne babban “buffer” na jiki.
Don haka, maida hankalinsu a cikin jini yana daidai da pH. Yawanci kodan ne ke daidaita yawan adadin bicarbonates na jini, yana haɓaka riƙewar su ko fitar da su.
Don tsara pH, da bicarbonate ion HCO3- hade da H ion+ don ba da ruwa da CO2. Matsayin kasuwancin jari na CO2 a cikin jini na arterial (Pa CO2), ko capnia, ko wani sashi na matsin lamba da CO2 ke yi a cikin jinin jijiya, don haka ma alama ce ta ma'aunin acid-base. Ana auna shi yayin nazarin iskar gas.
Bicarbonate ions ne na asali: lokacin da maida hankali ya karu, pH kuma yana ƙaruwa. Sabanin haka, lokacin da maida hankalinsu ya ragu, pH ya zama acidic.
A cikin mutum mai lafiya, pH na jini yana da ƙarfi sosai: 7,40 ± 0,02. Kada ya sauke ƙasa da 6,6 ko tashi sama da 7,7, wanda bai dace da rayuwa ba.
Me yasa yin nazarin bicarbonate?
Matsakaicin ions bicarbonate yana ba da damar kimanta ma'aunin acid-base na jini. Ana aiwatar da shi a lokaci guda tare da nazarin iskar jini, lokacin da likita ya yi zargin kasancewar rashin daidaituwa na tushen acid (acidosis ko alkalosis). Wannan na iya zama lamarin idan akwai wasu alamomi, kamar:
- canza yanayin hankali
- hypotension, ƙananan fitarwa na zuciya
- cututtuka na numfashi (hypo- ko hyperventilation).
- Ko kuma a cikin yanayi mara kyau kamar rashin narkewar abinci na al'ada ko asarar fitsari ko rikicewar electrolyte.
Binciken bicarbonates
Gwajin jinin ya ƙunshi samfurin jini na venous, yawanci a ninkan gwiwar hannu. Ba shiri ya zama dole.
Menene sakamakon za mu iya tsammanin daga nazarin bicarbonates?
Binciken ya sa ya yiwu a tantance kasancewar acidosis ko a alkalosis. Ma'aunin pH zai ba ku damar ganin idan akwai hyperacidemia (wanda aka bayyana azaman pH a ƙasa 7,35) ko hyperalcalemia (ƙimar pH sama da 7,45).
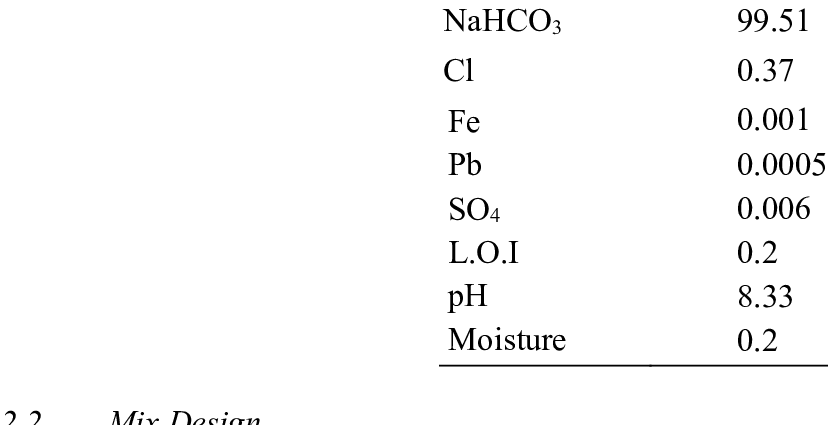
Ma'auni na ions bicarbonate da PaCO2 sa'an nan kuma ya ba da izini don ƙayyade idan rashin lafiya ya kasance na asali na rayuwa (rashin haɓaka na bicarbonates) ko numfashi (rashin lafiya na PaCO).2). Ma'auni na al'ada don bicarbonates shine tsakanin 22 zuwa 27 mmol / l (millimoles a kowace lita).
Rage yawan adadin ion bicarbonate da ke ƙasa da ƙimar al'ada yana haifar da acidosis na rayuwa. Acidosis yana da alaƙa da wuce haddi na H + ions. A cikin yanayin acidosis na rayuwa, za a sami raguwa a cikin maida hankali na ions bicarbonate (pH <7,35). A cikin acidosis na numfashi, shine karuwa a cikin matsa lamba na CO2 wanda zai zama alhakin karuwar H + ions.
Metabolic acidosis na iya zama saboda, a tsakanin sauran abubuwa, zuwa ga rashin daidaituwa na asarar bicarbonates saboda gudawa ko jiko saline na physiological.
Sabanin haka, karuwa a cikin maida hankali na ions carbonate yana haifar da a metabolism alkalosis (pH> 7,45). Yana iya faruwa a cikin taron na wuce kima gudanar da bicarbonates, amai mai tsanani ko asarar potassium (diuretics, zawo, amai). Hakanan ana iya haɗa hyperaldosteronism (hypersecretion na aldosterone).
Alkalosis na numfashi, a nasa bangare, yayi daidai da raguwar raguwa a cikin matsa lamba na CO2.
Karanta kuma: Duk game da hypotension |